
This understanding will then be molded into a few guidelines that should help pave the way for future developments of 2D polymers by those interested in joining the effort with this fascinating and emerging class of 2D materials.Ī. Consequently, the reader will understand why some crystals break during polymerization, while others stay intact. The very heart of both techniques will be explained and it will be shown what can be safely concluded with their help and what not. The article will show that single crystal X-ray diffraction based on both Bragg and diffuse scattering are powerful techniques to achieve such goal.
X ray diffraction pattern of polymers how to#
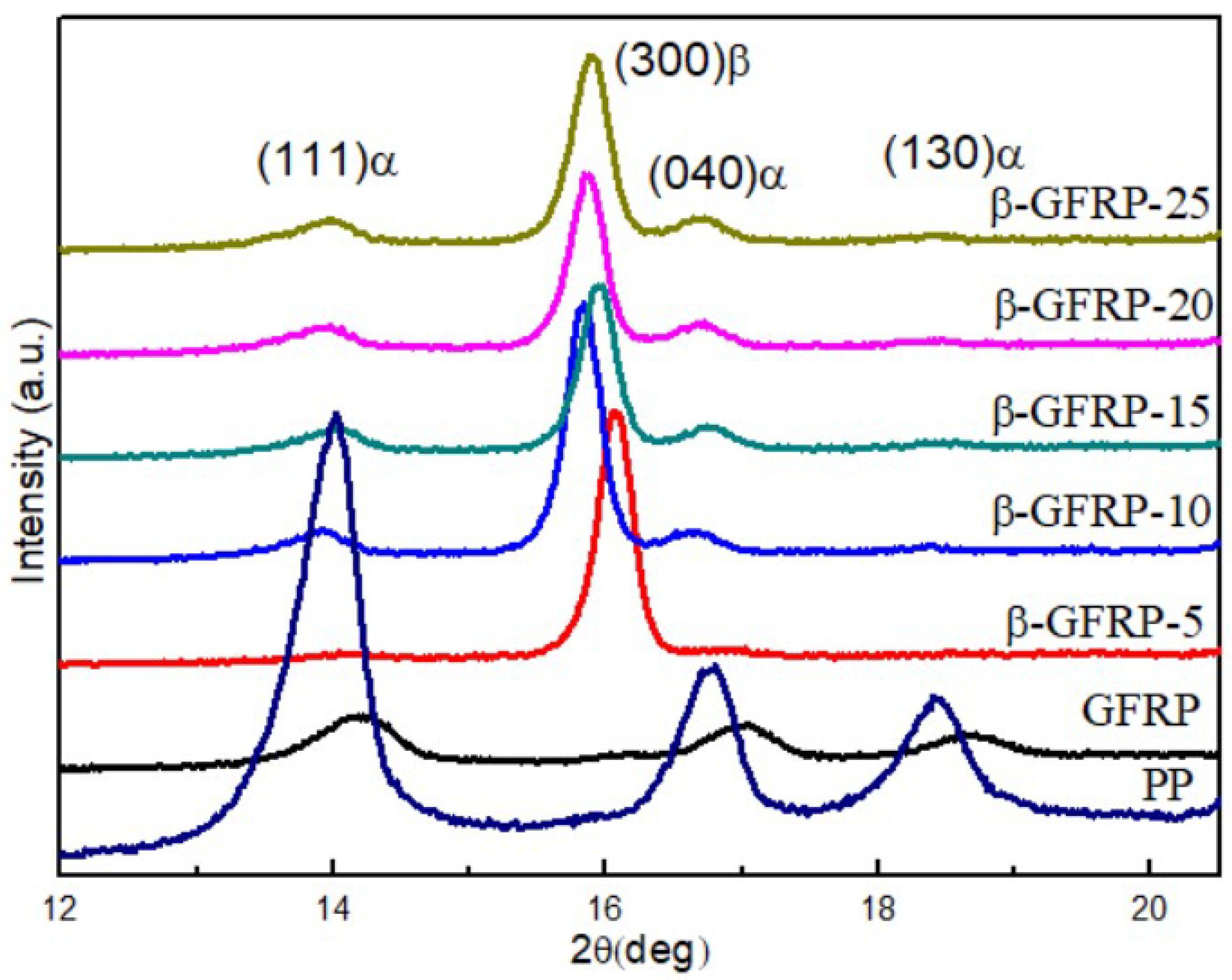
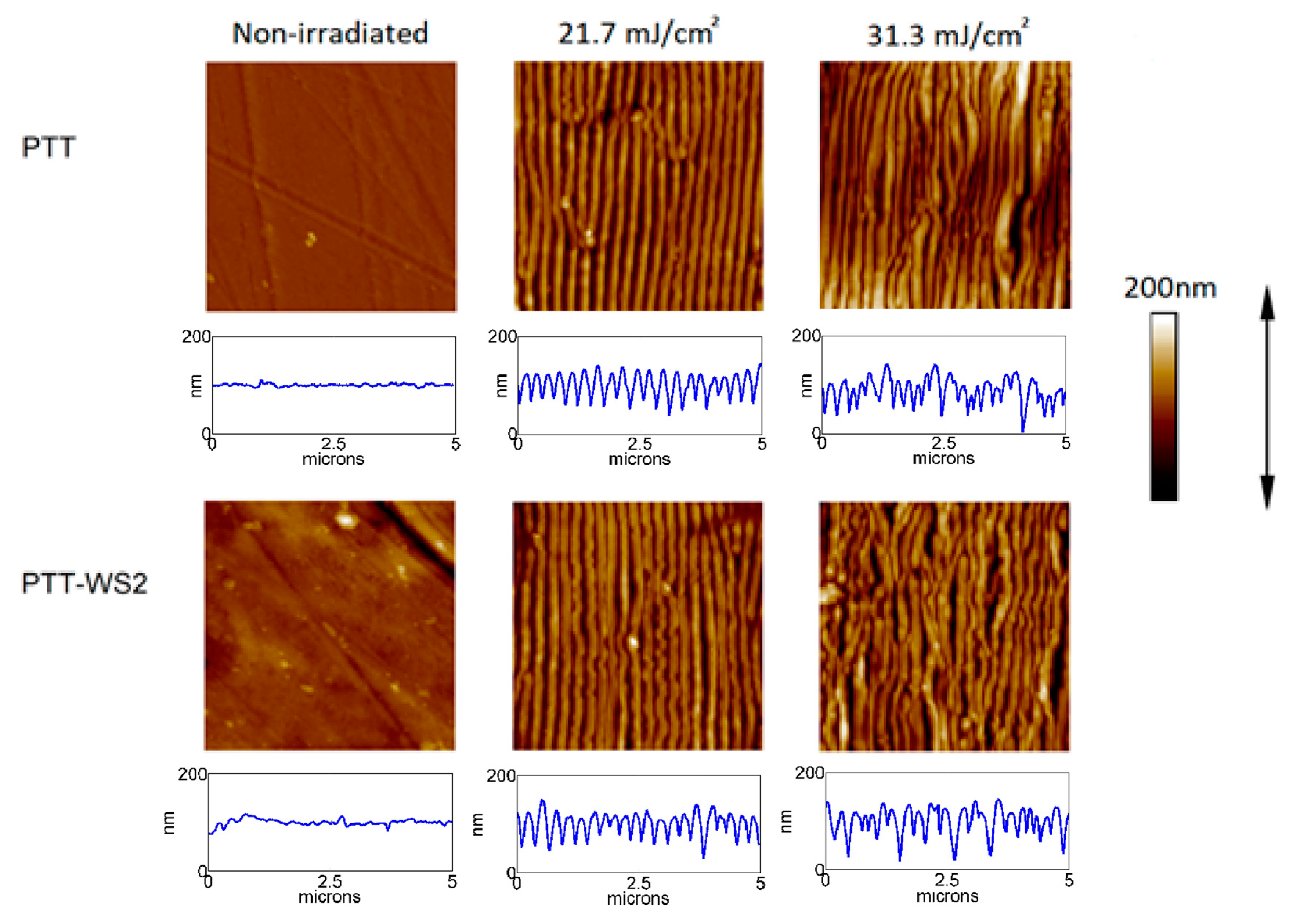
This raises questions as to how to elucidate the mechanism of these unusual polymerizations as well as their entire strain management. To have such an action proceed successfully, billions of bond formation processes have to be mastered exclusively in two dimensions within 3D crystals. However, since phase separation has occurred, the solid nanosuspensions would be expected to exhibit a greater tendency for physical instability under a given stress, that is, crystallization, than would a miscible system.Covalent long-range ordered (crystalline) sheets called 2D polymers have recently been synthesized by irradiating single crystals of suitably packed monomers. Such systems would be expected to have properties intermediate to those observed for miscible and macroscopically phase separated amorphous dispersions. Since DSC can not detect two T(g) values when phase separation produces amorphous domains with sizes less than approximately 30 nm, it is concluded that the trehalose-dextran system is a phase separated mixture with a structure equivalent to a solid nanosuspension having nanosize domains.
X ray diffraction pattern of polymers pdf#
In the case of the trehalose-dextran mixture, where only one T(g) value was detected, however, PDF analysis clearly revealed phase separation. From the PDF analysis, indomethacin-PVP was shown to be completely miscible in agreement with the single T(g) value measured for the mixture. In agreement with DSC measurements that detected two independent T(g) values for the dextran-PVP mixture, the PDF profiles of the mixture matched very well indicating a phase separated system. A lack of agreement of the PDF profiles indicates that the mixture with a unique PDF is miscible. Immiscibility is detected when the PDF profiles of each individual component taken in proportion to their compositions in the mixture agree with the PDF of the mixture, indicating phase separation into independent amorphous phases. The mixtures chosen were: dextran-poly(vinylpyrrolidone) (PVP) and trehalose-dextran, both prepared by lyophilization and indomethacin-PVP, prepared by evaporation from organic solvent. Recognizing limitations with the standard method of determining whether an amorphous API-polymer mixture is miscible based on the number of glass transition temperatures (T(g)) using differential scanning calorimetry (DSC) measurements, we have developed an X-ray powder diffraction (XRPD) method coupled with computation of pair distribution functions (PDF), to more fully assess miscibility in such systems.


 0 kommentar(er)
0 kommentar(er)
